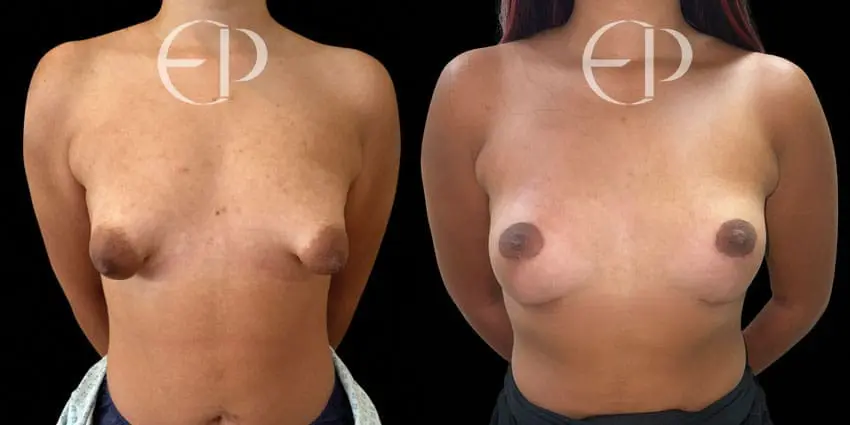
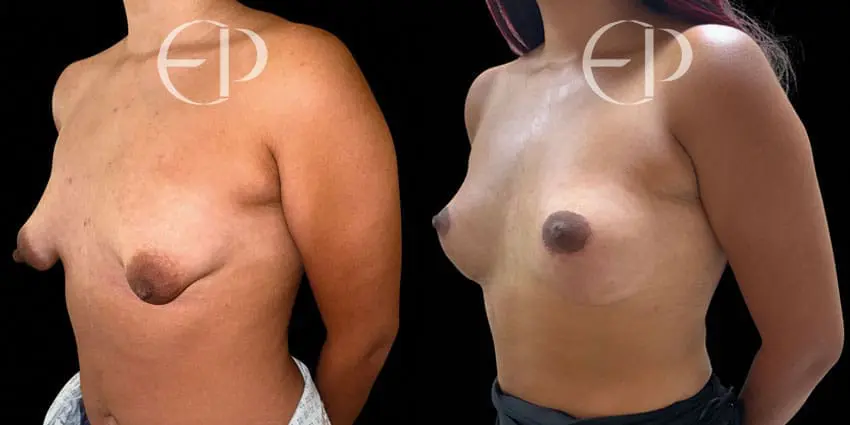
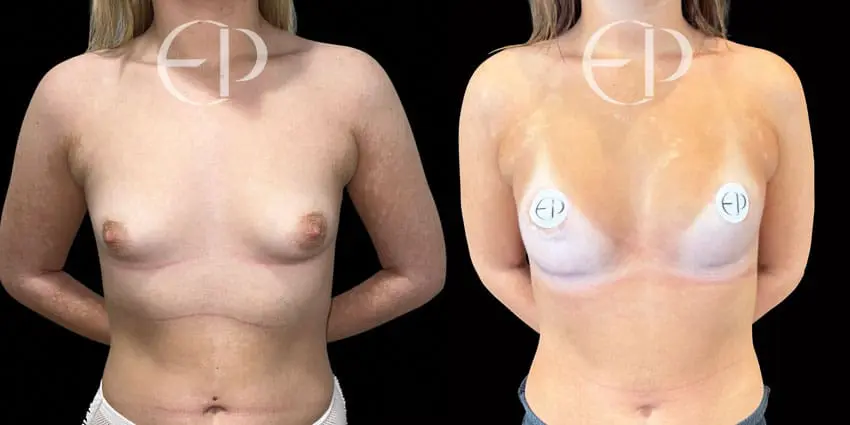
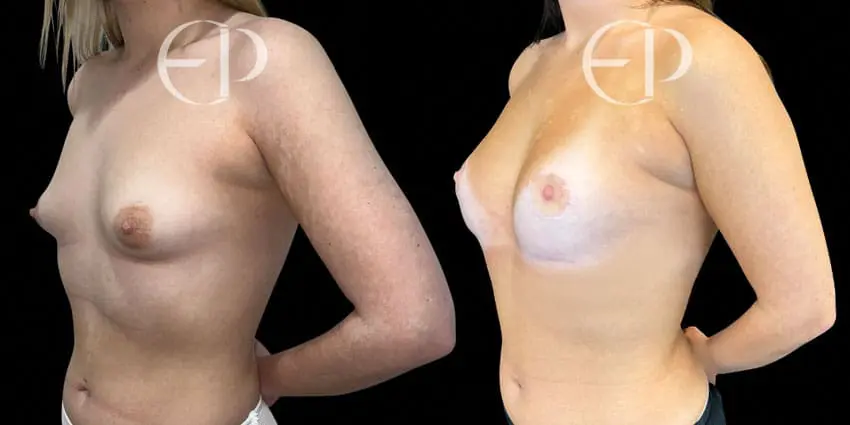
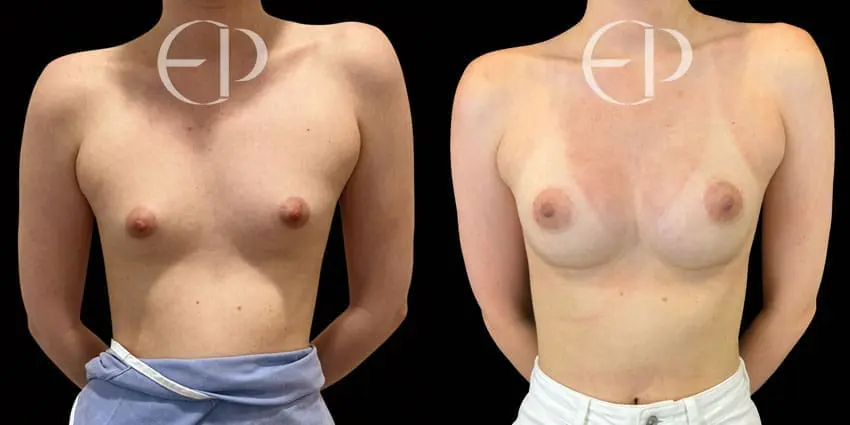
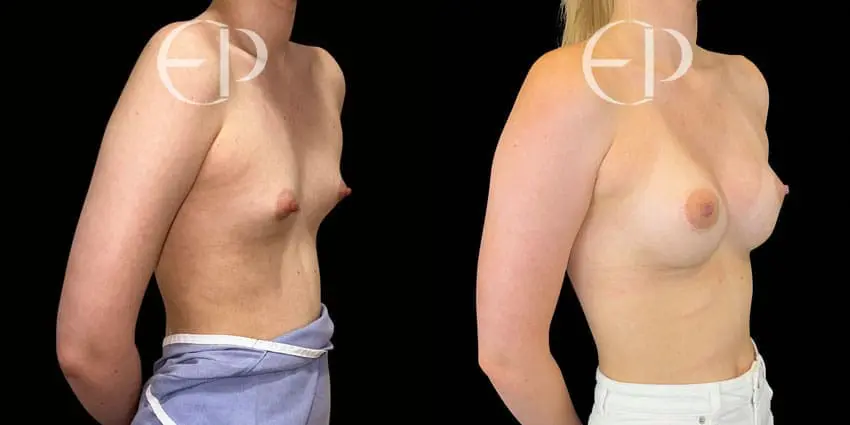
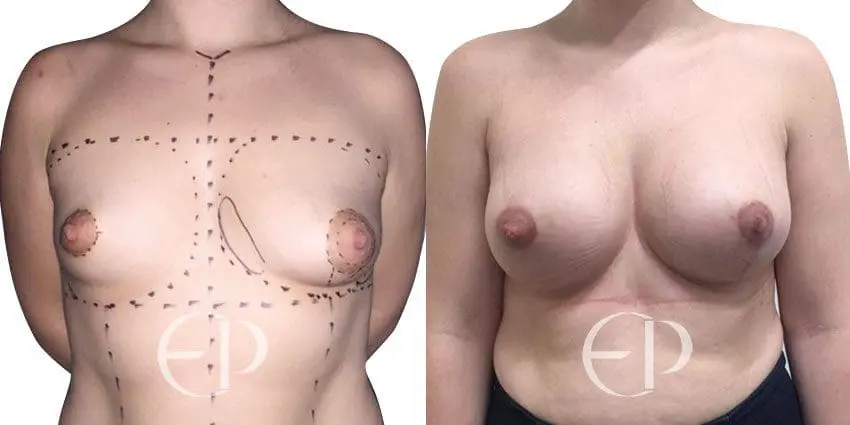
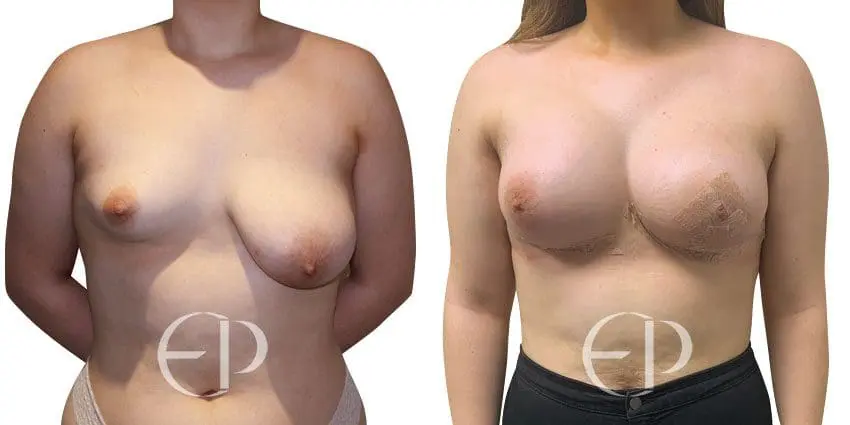
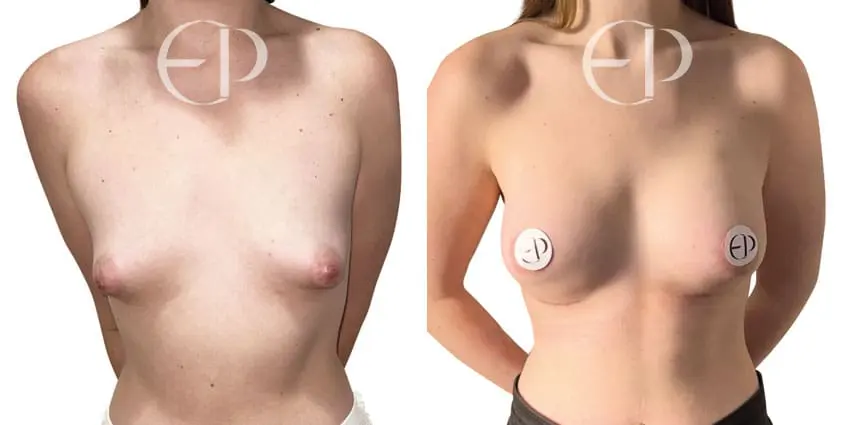
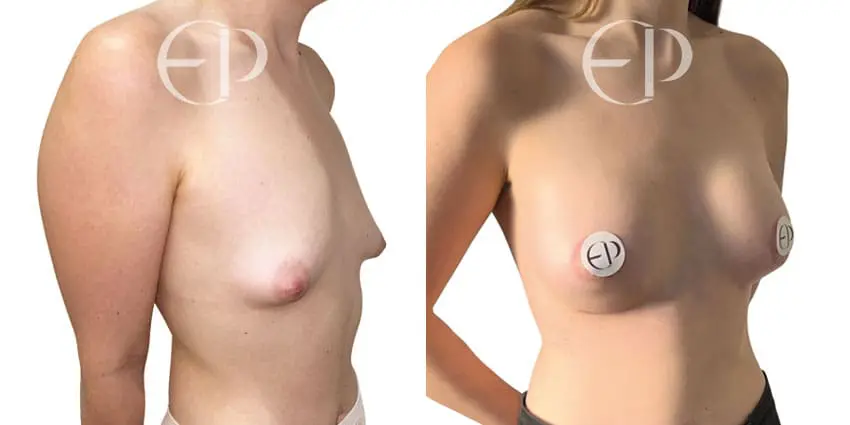
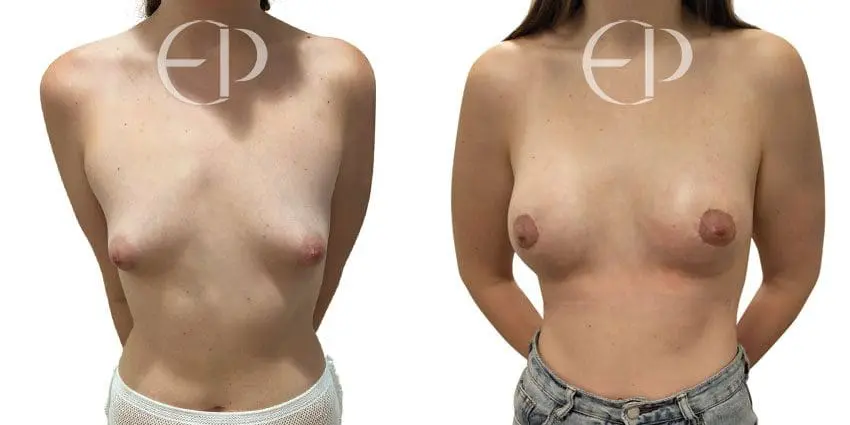
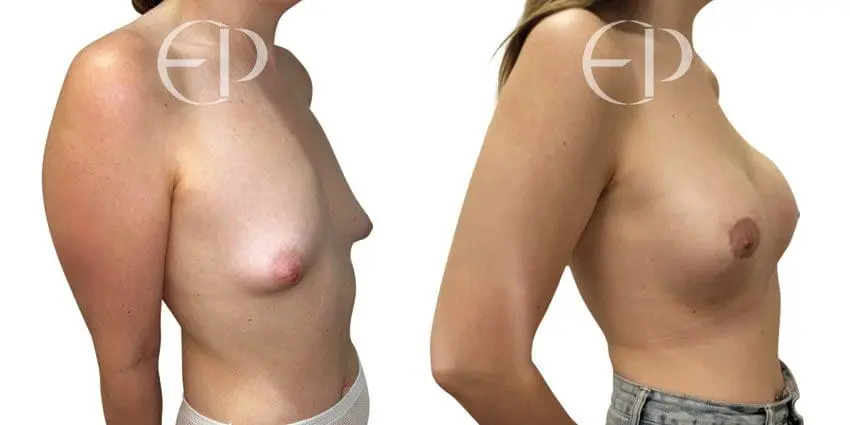
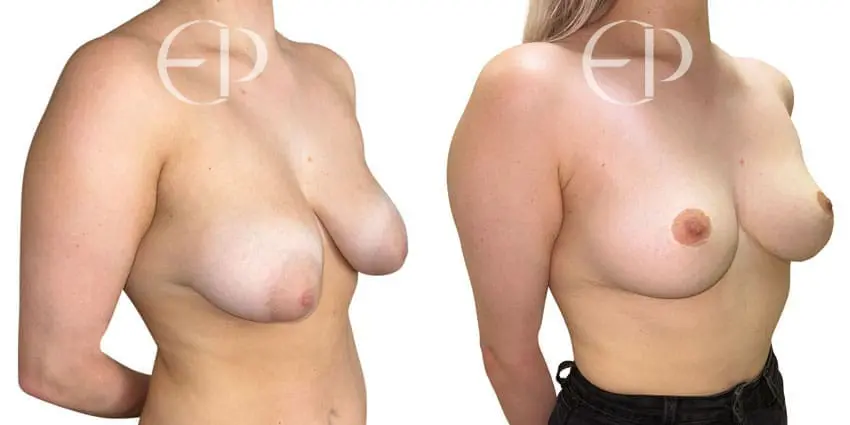
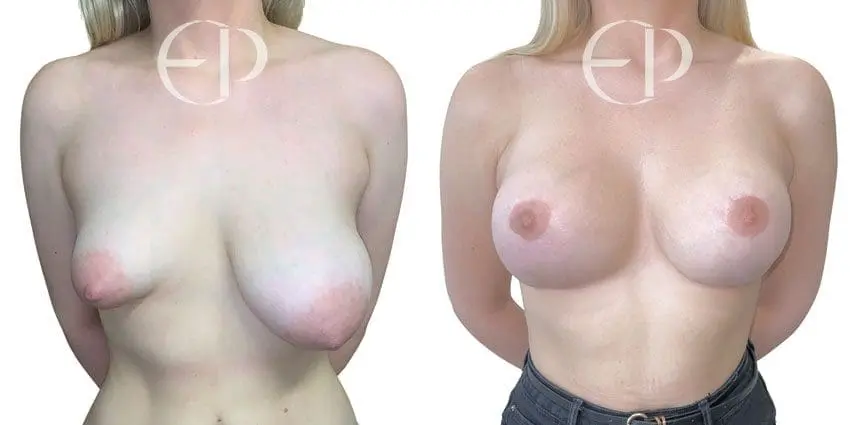
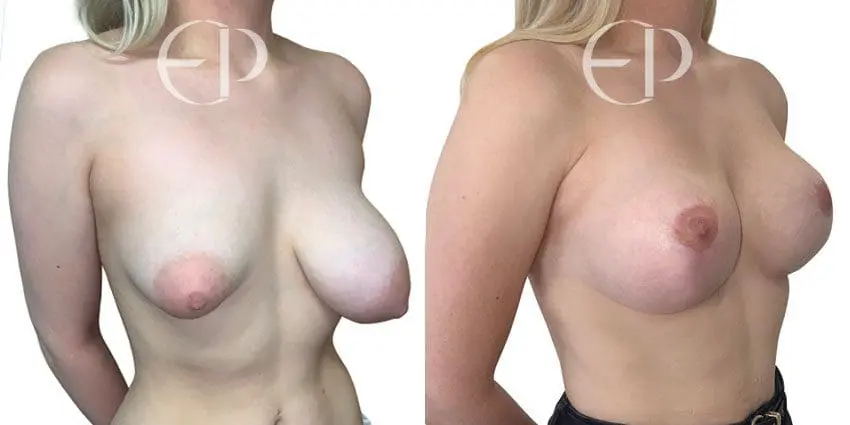
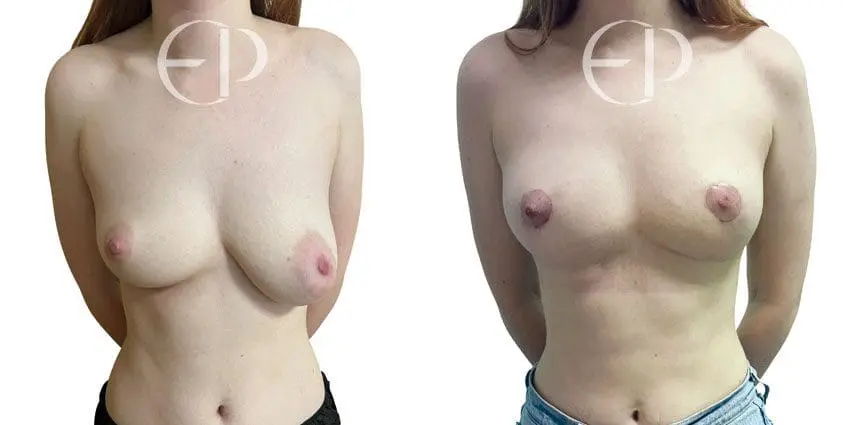
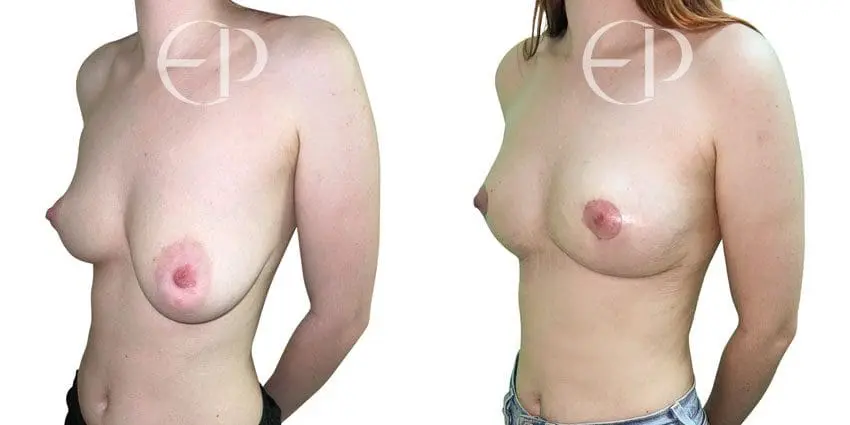
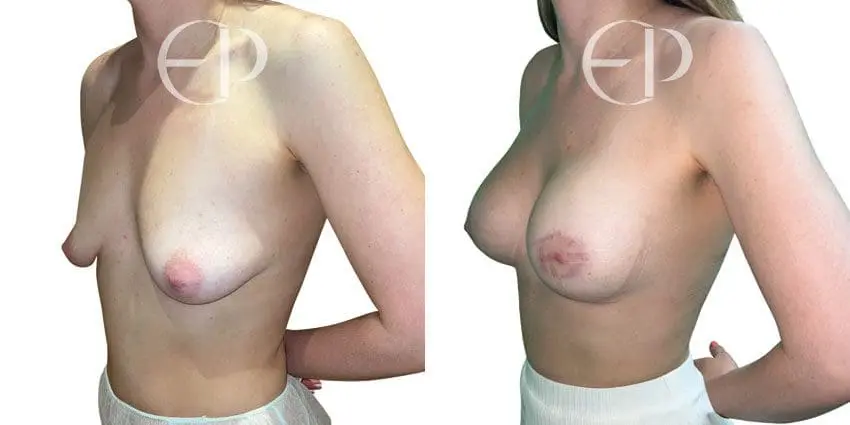
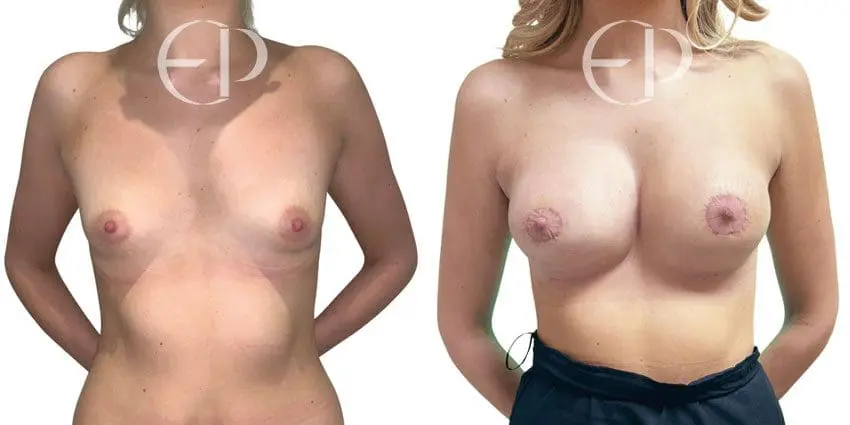
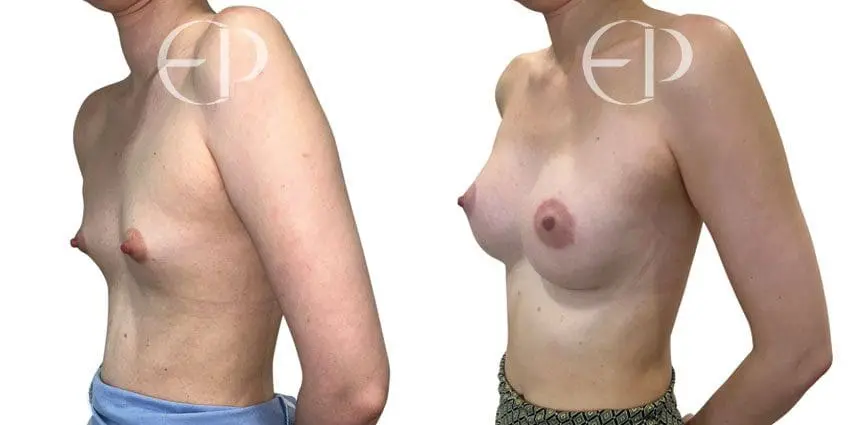
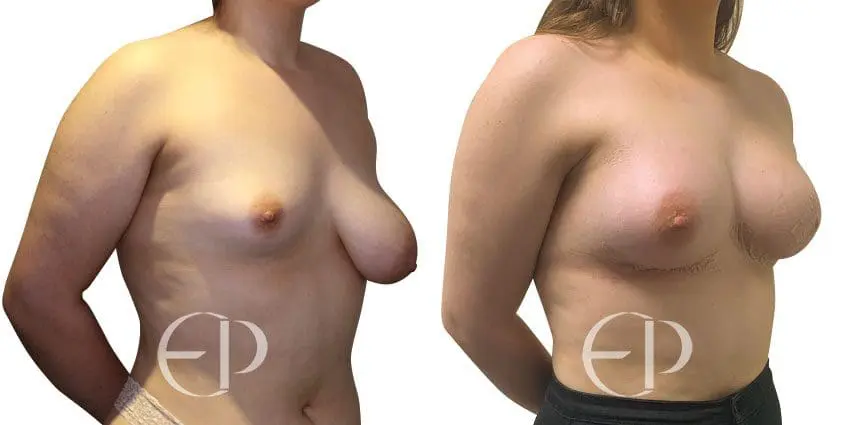
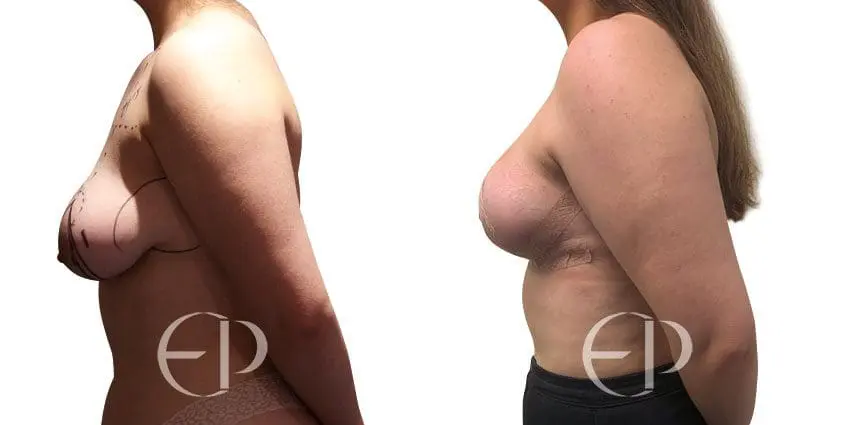
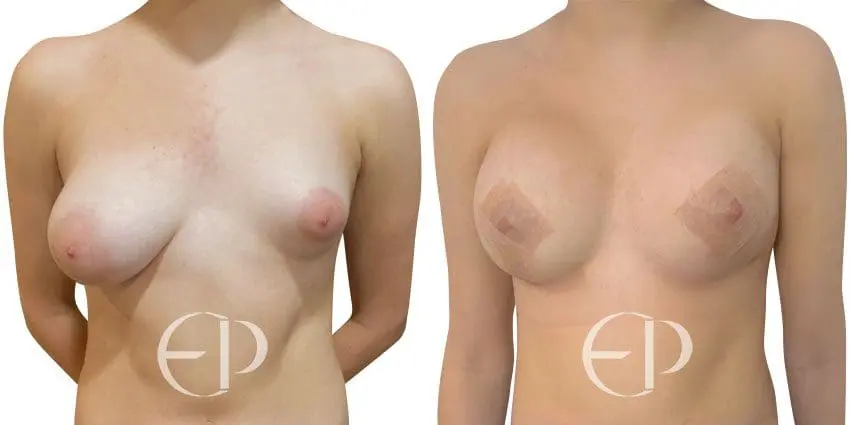
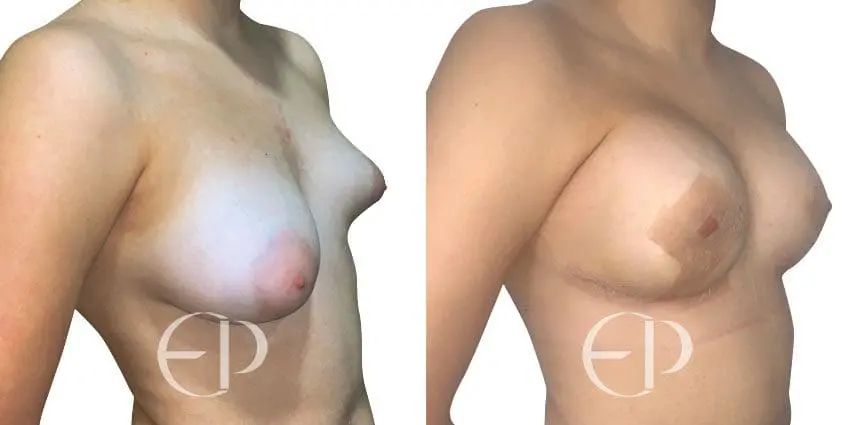
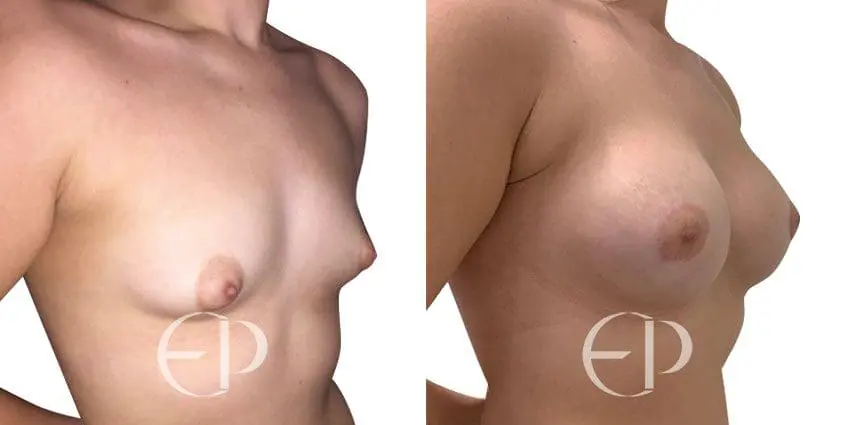
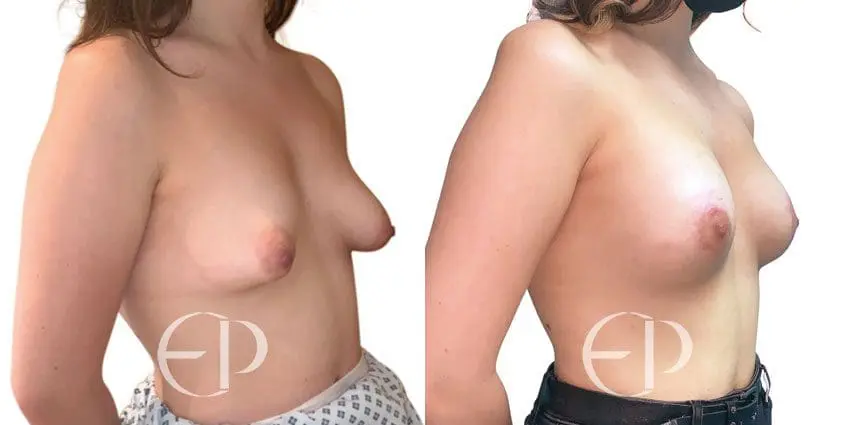
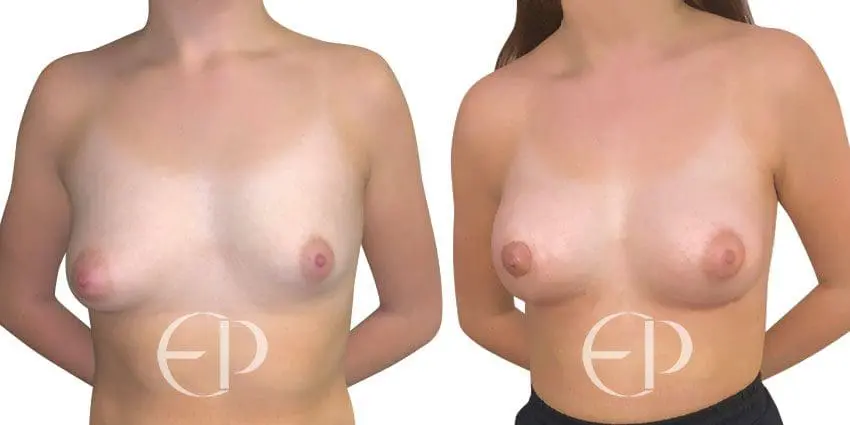
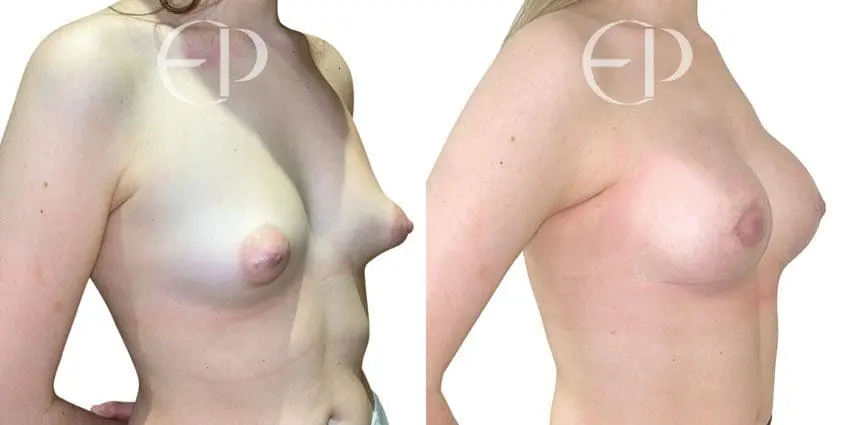
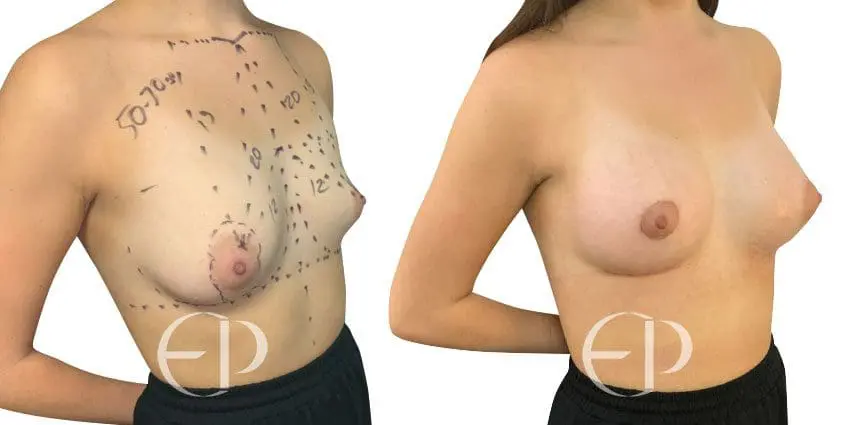
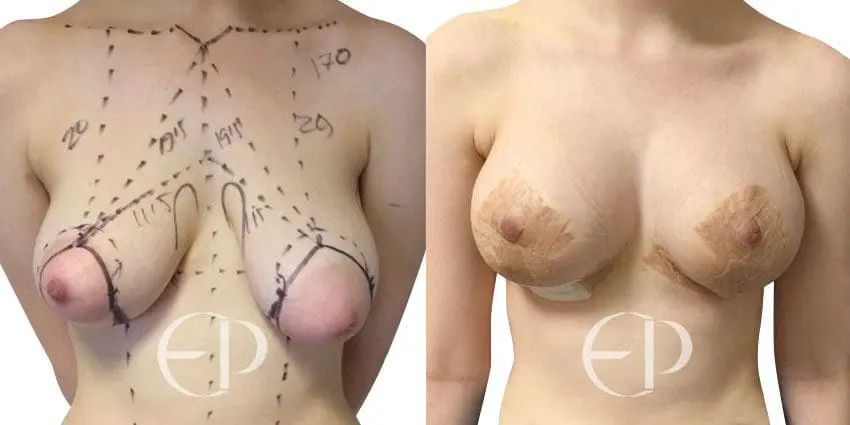
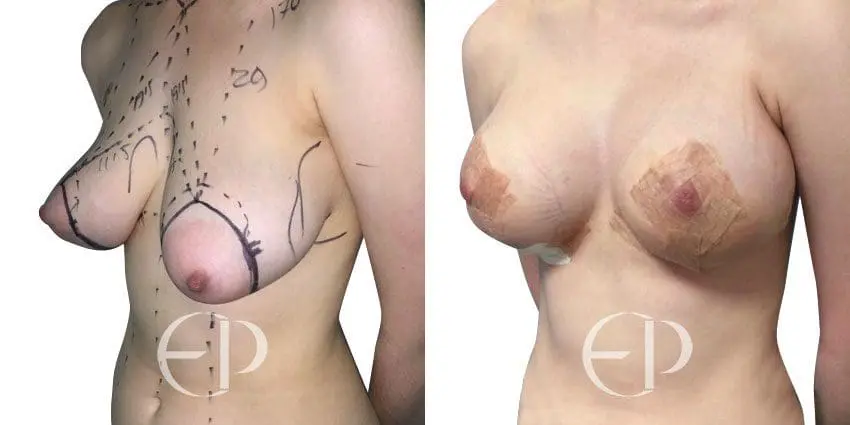
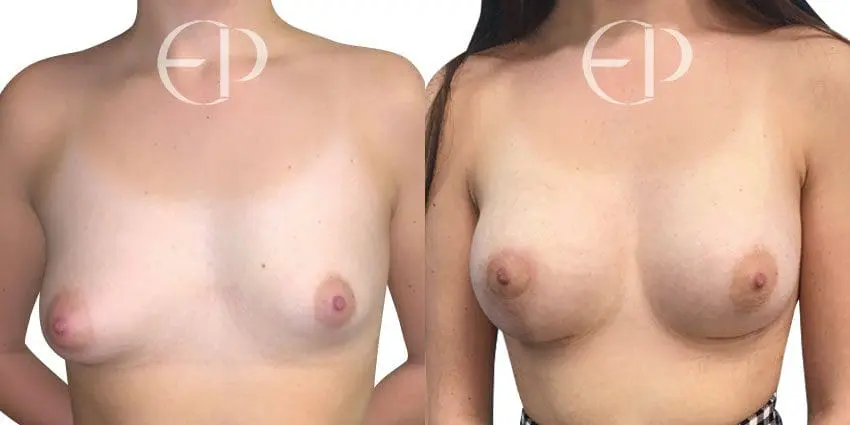
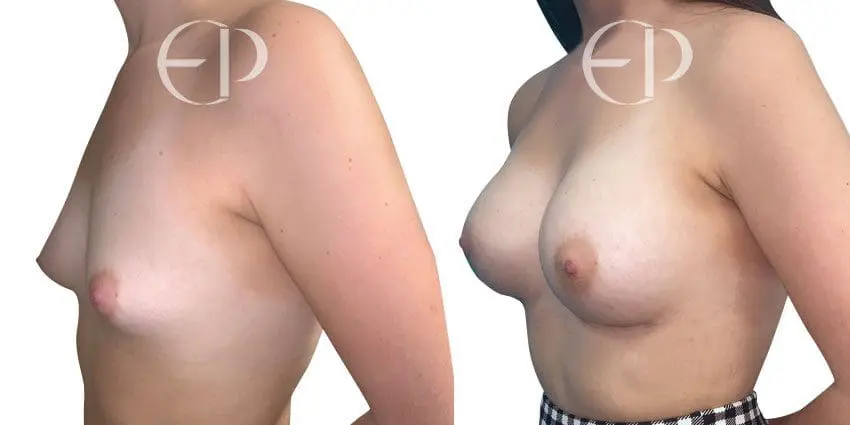
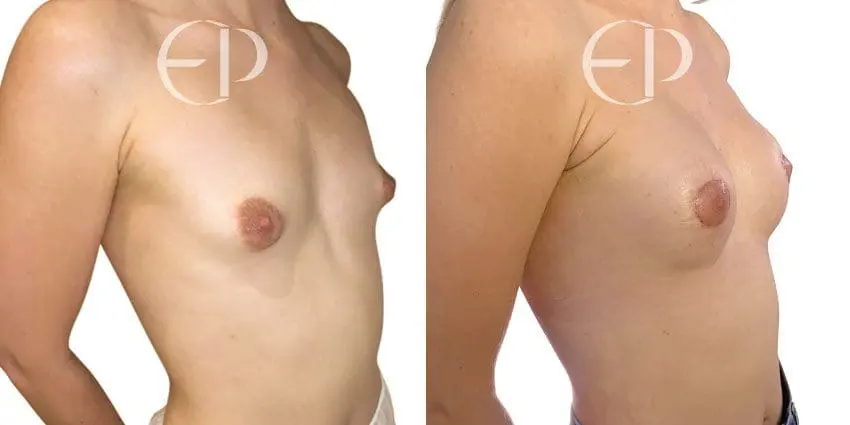
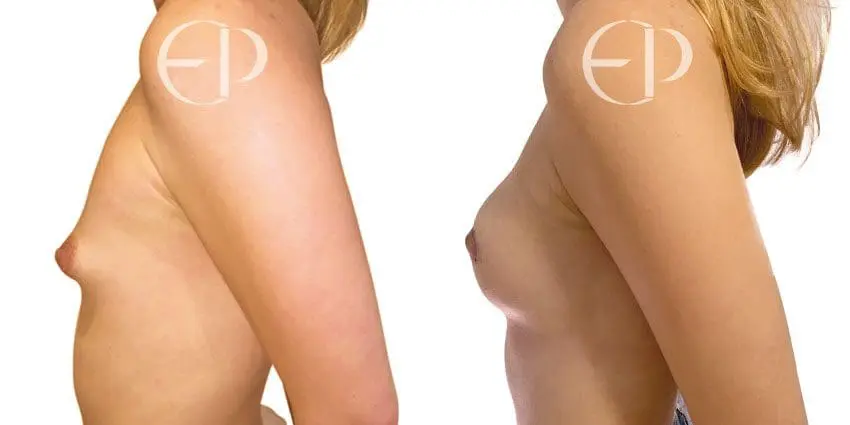
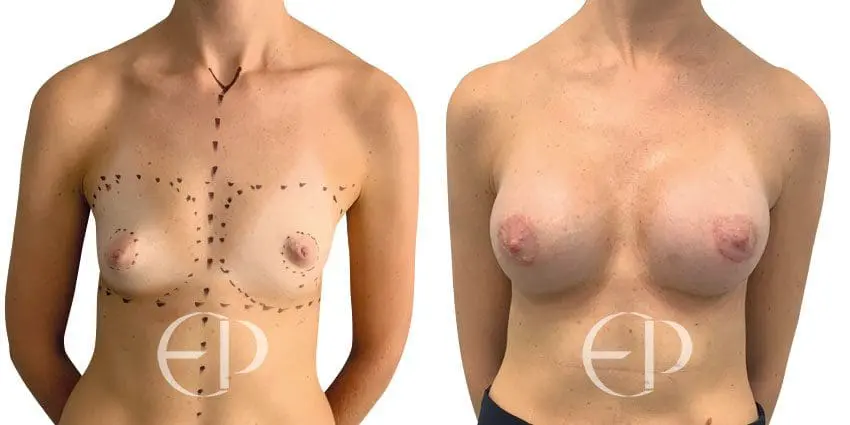
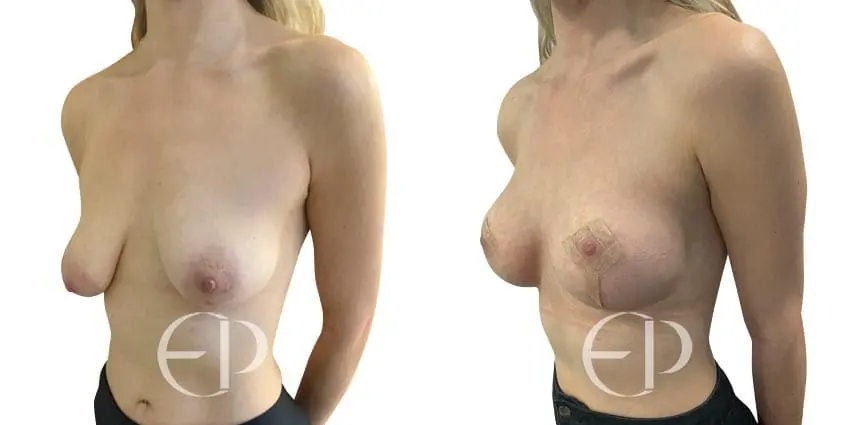
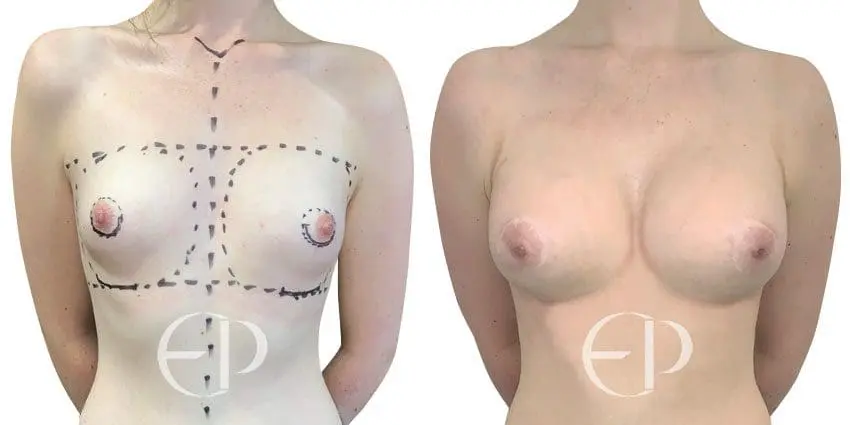
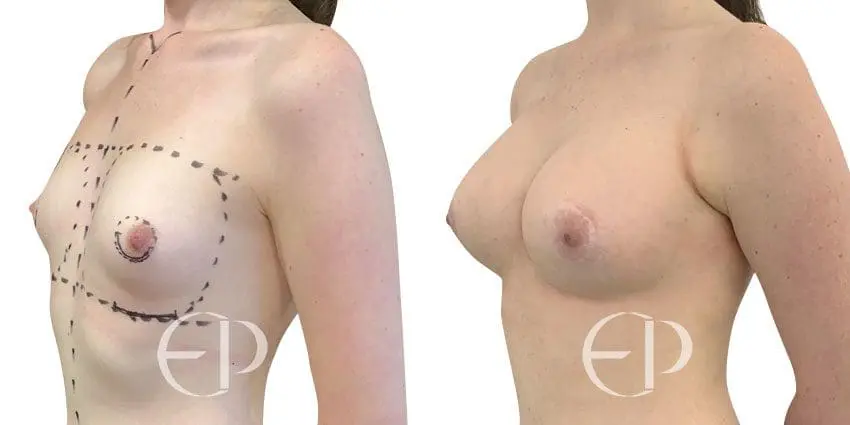
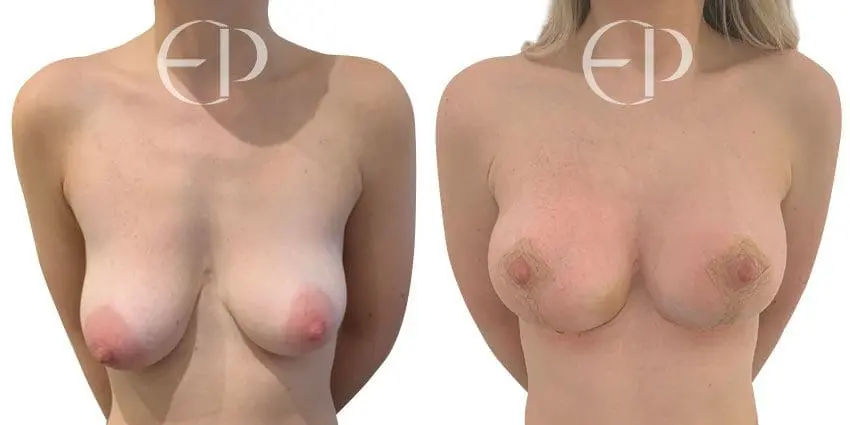
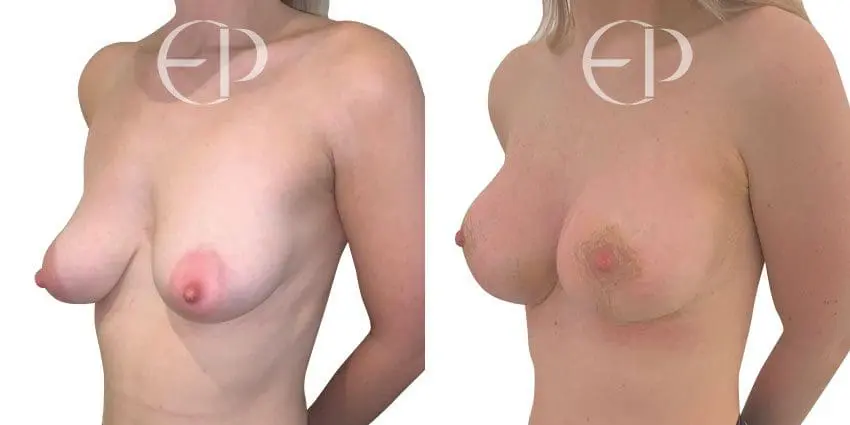
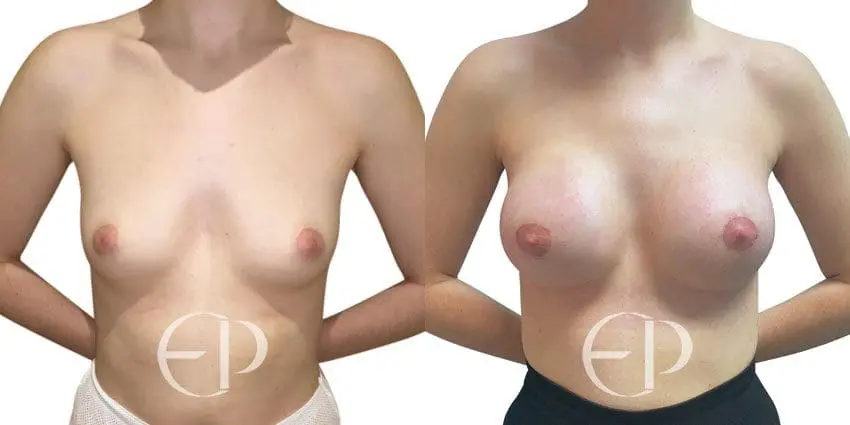
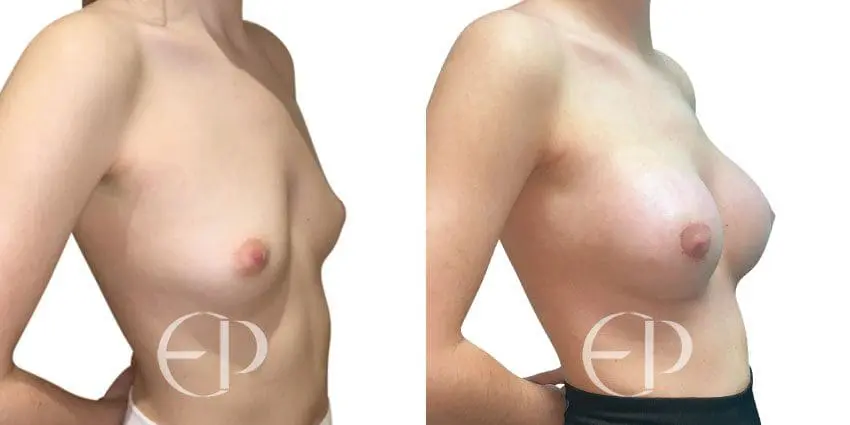
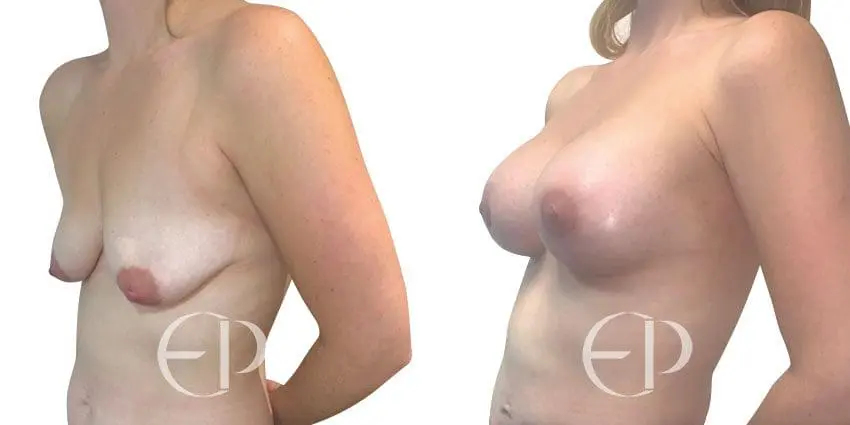
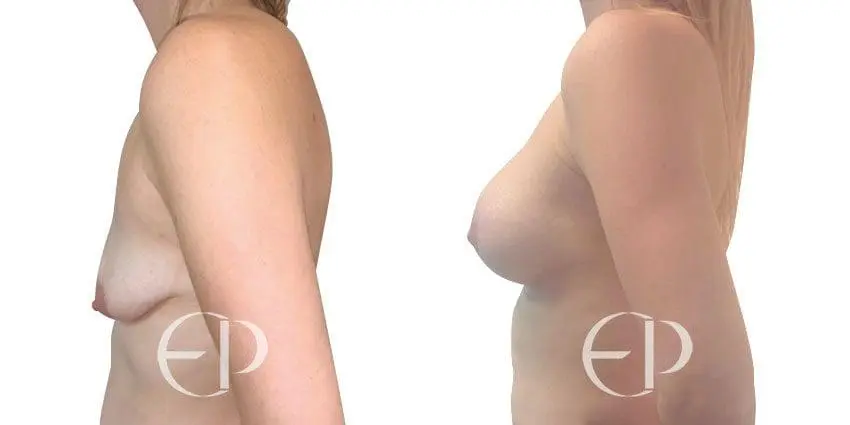
Since December 2018, many regulatory bodies around the World have issued statements regarding their own position and some have limited the distribution of other textured devices. The advice does vary from country to country because the research data available is limited and not conclusive. None have called for the precautionary removal or replacement of textured devices that are already inside patients’ breasts.
Breast implants however are not lifelong devices and in general will need removal or replacement at some point. In an effort to have more robust data about both conditions, the Breast and Cosmetic Implant register (BCIR) collects breast implant data for patients in England and Scotland. The MHRA continues to collect and analyse UK information through a reporting system. It also has links with up to date information from international public health organisations. The most recent advice from MHRA on BIA-ALCL was updated on the 4th April 2019.
View the MHRA statement on Allergan
Download the BAAPS statement on ALCL